[Explained] How Many Valence Electrons Does a Conductor Generally Have?
Have you ever wondered why some materials like copper and aluminum conduct electricity so efficiently, while materials like plastic and wood are poor conductors? The secret lies in the number of valence electrons of the material. Typically, for a material to qualify as a conductor, it should possess three or fewer valence electrons.
In this article, we will take a deep dive into the fascinating world of electrical conductors and uncover how their valence electrons enable the flow of electric currents.
What is a Valence Electrons?
Before jumping into the specifics of conductors, let’s first build a foundation by understanding what valence electrons are. In the structure of an atom, electrons occupy different shells or energy levels, with the outermost shell being the highest energy level. The electrons present in this outermost shell are called valence electrons.

These valence electrons play a crucial role in determining the chemical properties of an element. They are the electrons that participate in chemical bonds with other atoms. Valence electrons are also the electrons that can be “freed” most easily to allow electric current to flow. The number of valence electrons depends on the element’s position on the periodic table.
Conductors and Their Valence Electron
Conductors have a small number of valence electrons because they are more likely to lose these electrons and create a flow of electricity. Conductors generally have three or fewer valence electrons.
When a voltage is applied to a conductor, the valence electrons are free to move around inside the material. This creates an electric current.
Some common examples of conductors include:
- Metals, such as copper, aluminum, and iron
- Alloys, such as brass and bronze
- Electrolytes, such as salt water and battery acid
Conductors vs. Insulators
To comprehend the valence electrons in conductors, we should first distinguish them from insulators. Conductors are materials that allow electric current to flow through them easily. Metals are excellent examples of conductors. Insulators, on the other hand, greatly resist the flow of electric current. Rubber, glass, and wood are common insulators.
This difference in conductivity arises because conductors have valence electrons that are loosely bound to their parent atom, whereas insulators have tightly bound electrons. When an electric voltage is applied, the loosely bound electrons in conductors can easily break away and flow as electric current.
Valence Electrons in Metals
Metals are renowned for their excellent conductive properties. Their outermost electrons are loosely bound and can detach to become mobile when electric potential is applied. This is owing to metals having 1, 2, or 3 valence electrons in their atoms.
For example:
- Copper has 1 valence electron
- Magnesium has 2 valence electrons
- Aluminum has 3 valence electrons
These metals have a sea of free, mobile electrons that results in high conductivity. However, not all metals are used as conductors like Bismuth, and Mercury due to their low conductivity.
Valence Electrons in Superconductors
Superconductors are materials that exhibit zero electrical resistance below a characteristic critical temperature. They allow electric current to flow without any loss of energy. This remarkable property results from electrons binding together in pairs called Cooper pairs, which can move through the material without scattering.
So, while valence electrons are responsible for conduction in normal conductors, superconductivity relies on the coordinated motion of Cooper pairs. Examples of superconductors are mercury, niobium, and lead.
Electrical Conductivity of Material
The electrical conductivity of a material can be calculated using the following equation:
σ = neμ
where:
- σ is the electrical conductivity
- n is the number of charge carriers per unit volume
- e is the elementary charge
- μ is the mobility of the charge carriers
The mobility of the charge carriers is a measure of how easily they can move through the material.
Factors Affecting Conductivity
While the number of valence electrons has a big influence, some other factors also affect the conductivity of a material:
- Temperature: In metals, increased temperature causes increased vibrations of atoms, impeding electron flow. In semiconductors and superconductors, elevated temperature enables greater conductivity.
- Imperfections and Impurities: Defects in the material structure can hamper the easy flow of electrons. Doping with select impurities can improve conductivity.
- Applied Voltage: A higher potential difference causes more energetic acceleration of electrons, improving conductivity.
How to Find Valence Electrons
The valence electron count of any element can be easily deduced from its position in the periodic table. Elements belonging to the same group have identical valence electron configurations. For example:
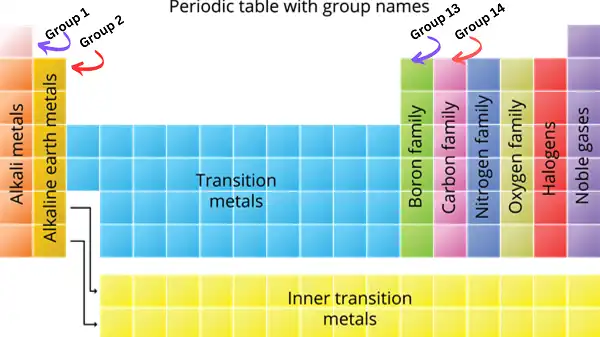
- Group 1 elements (alkali metals) have 1 valence electron
- Group 2 elements (alkaline earth metals) have 2 valence electrons
- Group 13 elements (boron group) have 3 valence electrons
- Group 14 elements (carbon group) have 4 valence electrons
And so on. This pattern helps determine the expected conductivity of any element.
Real-World Applications
The free flow of electrons makes conductors indispensable for various practical applications:
- Electrical wiring: Metallic wiring in households and circuits relies on conductor properties to deliver electricity.
- Electronics: Semiconductors are crucial components of diodes, transistors, and integrated circuits enabling modern electronics.
- Superconducting magnets: These are used to generate extremely high magnetic fields for applications like MRI machines and particle accelerators.
- Research: Properties of conductors and superconductors continue to be studied for insights into quantum mechanics and particle physics.
Frequently Asked Questions (FAQs)
1. Can Lowering Temperature Turn a Regular Conductor into a Superconductor?
Answer: Yes, some metals and alloys exhibit zero resistivity at very low cryogenic temperatures as their electrons form Cooper pairs and flow without impediment.
2. Why Do High-Voltage Power Lines Need More Robust Insulation Than Household Wires?
Answer: High-voltage power lines need insulation that can withstand the larger electric field without allowing electrons to get pulled off the insulator molecules and start conducting current.
3. What Happens if a Conductor Is Overloaded with Current?
Answer: If a conductor is overloaded with excessive current, it can overheat and potentially lead to a fire or damage to the equipment. Conductors should always be sized correctly to handle the expected current load.
To Conclude
The number of valence electrons in a material is one of the most important factors that determines its electrical conductivity. This article enhances our understanding of the materials shaping our technological world but also opens doors to innovations in the field of electrical conductivity.
Subscribe to our newsletter
& plug into
the world of circuits